| # | Title | Image | Description | Video |
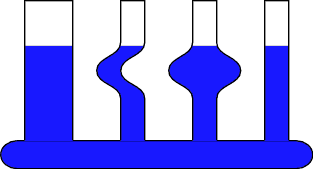
| 1 | Pascal's Vases |
Static Pressure
|
Water levels in different shaped containers attached to a common reservoir will reach the same height no matter what the shape of the container. |
|
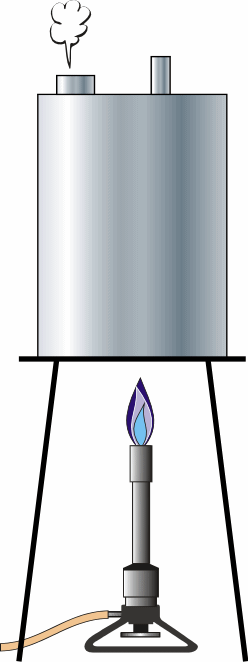
| 2 | Crush the Can |
Atmospheric Pressure
|
Pour an inch of water into the can. Set it on a stand and start the gas burner. Let it boil for a while to displace all the air. Use insulated gloves to tighten the cap onto the can and at the same time, turn off the heat. Spray it with water to cool it faster. If the cap is tight, and as the steam inside cools and contracts, the can will collapse from atmospheric pressure. |
|
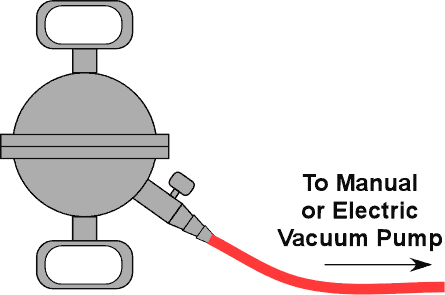
| 3 | Magdeburg Hemispheres |
Atmospheric Pressure
|
Make sure the valve is open. Turn on the vacuum pump to evacuate the hemispheres. Shut the valve and remove the hose. Have two students try to pull the hemispheres apart. You may also use your lungs to create a partial vacuum or a hand pump. |
|
| 4 | Manometers |
Measuring Pressure
|
Simple water and mercury manometers. |
|
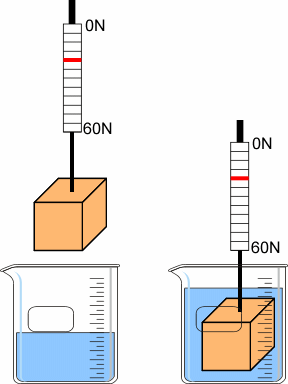
| 5 | Weigh Submerged Block |
Density and Buoyancy
|
Weigh the block in air, then submerged in water. The difference is the buoyant force. |
|
| 6 | Archimedes' Principle |
Density and Buoyancy
|
A cylindrical mass and bucket are suspended from a spring scale above a beaker with an overflow spout. Note the scale reading. Submerge the mass by raising the beaker with the lab jack. Pour the water from the catch bucket into the hanging bucket to return to the original scale reading. You may also show that the mass exactly fits inside the hanging bucket. |