Electric charges are all around us. They are responsible for many of the phenomena we see, including the true nature of the normal force. Some charges can move and some can reorient to create interesting effects.
Pre-lecture Study Resources
Watch the pre-lecture videos and read through the OpenStax text before doing the pre-lecture homework or attending class.
BoxSand Introduction
Electric Phenomena | Micro-model of Charge and Q-transfer
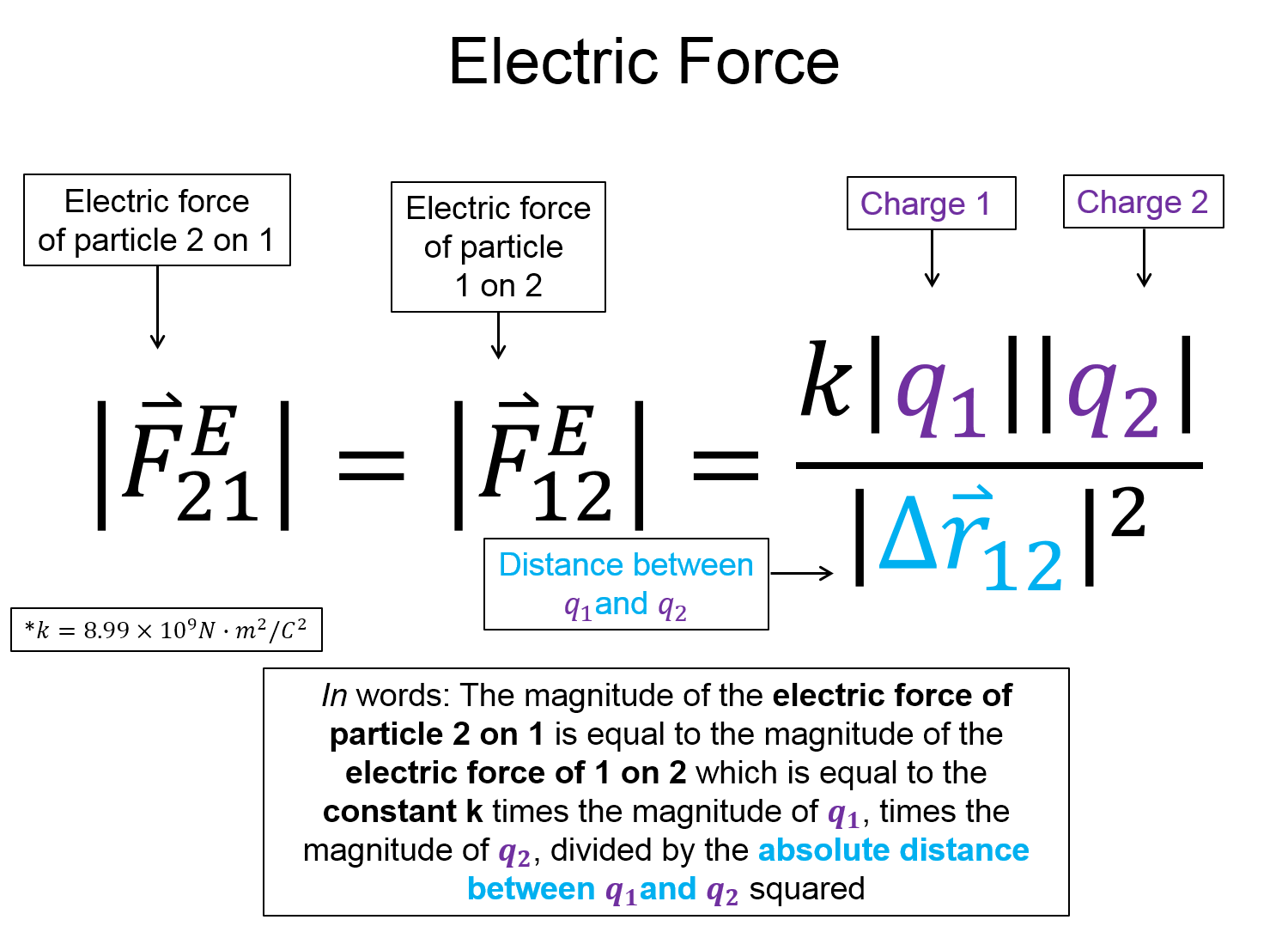
All of the forces we experience on a daily basis, except gravity, are due to the electric force. The electric force is a fundamental feature of charged particles. Everyday matter is largely comprised of these particles - the positively charged proton and negatively charged electron. If you're sitting in a chair, the force preventing you from falling towards the center of the Earth is due to repulsive force of the electrons on your bottom with the electrons on your seat. The electrons can't part ways and slide past each other, which would cause you to fall to the floor, because of their attraction to the protons in the nucleus. This balance of repulsion and attraction, is what causes all of the contact forces we experience (normal, friction, tension, buoyancy, etc.).
To understand how this works, we should start with some fundamental facts.
- Particles are either positive (protons), negative (electrons), or neutral (neutron).
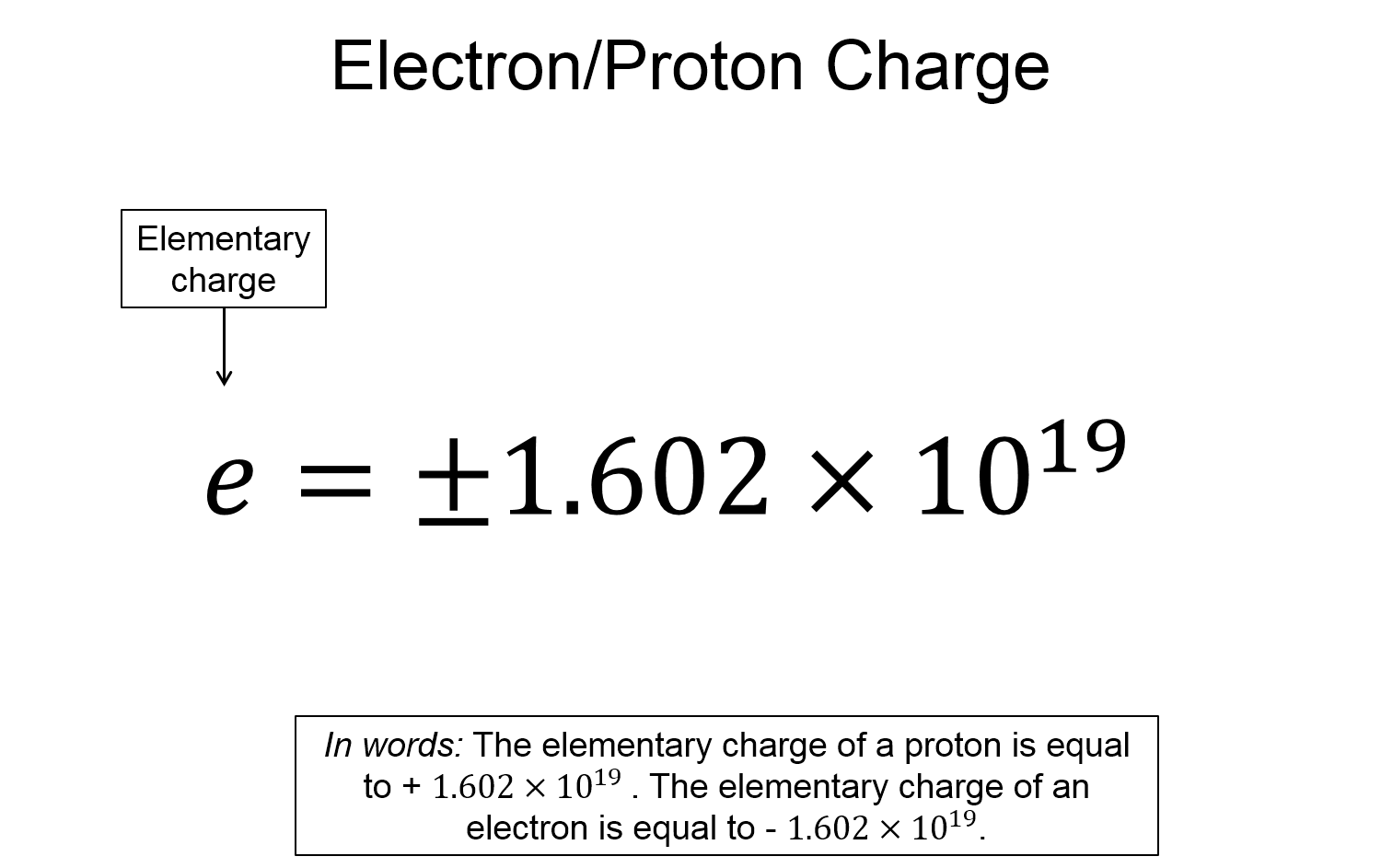
- Charges are quantized - the charge of a proton or electron is $e=\pm 1.602\times 10^{-19}$ coulombs.
- Total net charge of a system is, $Q_{net}=e(N_{protons}-N_{electrons})$, where N represents the number of protons or electrons.
- Like charges repel each other while unlike charges attract each other.
- Charges are transferable from one object to another.
- Insulators are material with no free electrons and thus charges cannot flow freely throughout the material. A Conductor is a material with free electrons (unbound) that can move freely, spreading out uniformly throughout the material's surface.
Conductors vs. Insulators
Most electrons are bound to a particular atom but there are some electrons that will be transferred to another atom or an entirely different object. Understanding how charge transfer works starts by understanding the two main types of materials, insulators and conductors. Both materials have fairly fixed positive ion cores (protons in the nucleus) that are much heavier (x1000) than the electrons surrounding them. Both have tightly bound electrons in the inner orbital shells.

Where they differ is in the presence of free electrons. Conductors have electrons that are bound enough to stay on the material but not bound too strongly to any one atom. They can move freely, allowing them to redistribute throughout an object quickly if initially concentrated in one location. Metals are often conductors and are used in electrical wiring. Insulators, in contrast, have no free electrons, and as such do not allow current to flow through them easily. The rubbery material surrounding the electrical wires are insulators to prevent you from getting shocked.
Charge Transfer
Electrons can be transferred between objects allowing for them to be in a net negative or net positive state. If two objects are initially net neutral, but after charge transfer occurs between them one is net positive, you know the other must be equally net negative. This is because one loses electrons and the other gains electrons. The protons (and neutrons) in the nucleus are not directly involved in any charge transfer, they are too far away and shielded from the action happening on the outskirts of the atom, where most of the important interactions occur.
Conductors can exchange charges by simply touching due to their free electrons. If touched, both objects will come to an equivalent equilibrium charge distribution. Consider two metal spheres, equivalent in every way except their charge, being brought into contact. If one was initially charged with a net charge $Q_{net}=+4 C$ and the other $Q_{net}=-2 C$, they have a net initial charge of $+2 C$. Since they have the same surface area, they will split the net charge equally, $+1 C$ each. The change will happen very rapidly (fractions of a fraction of a second), with the free electron charges transferring between objects and redistributing evenly. If the shape and size of the objects differ, they tend towards having the same surface charge density.
Insulators can have some charge transfer by simple contact but friction is often required for any large effects. Take a plastic rod and some cat fur and rub them together and one will lose electrons while the other gains them. Which one gains or loses an electron depends on the relative electronegativity of each material. Insulators differ from conductors in that the exchange of electrons only effects the region where the touching occurred. Since there are no free electrons to redistribute the excess charges, only the top half of the plastic rod would display charged effects if that was the only half rubbed.
Key Equations and Infographics
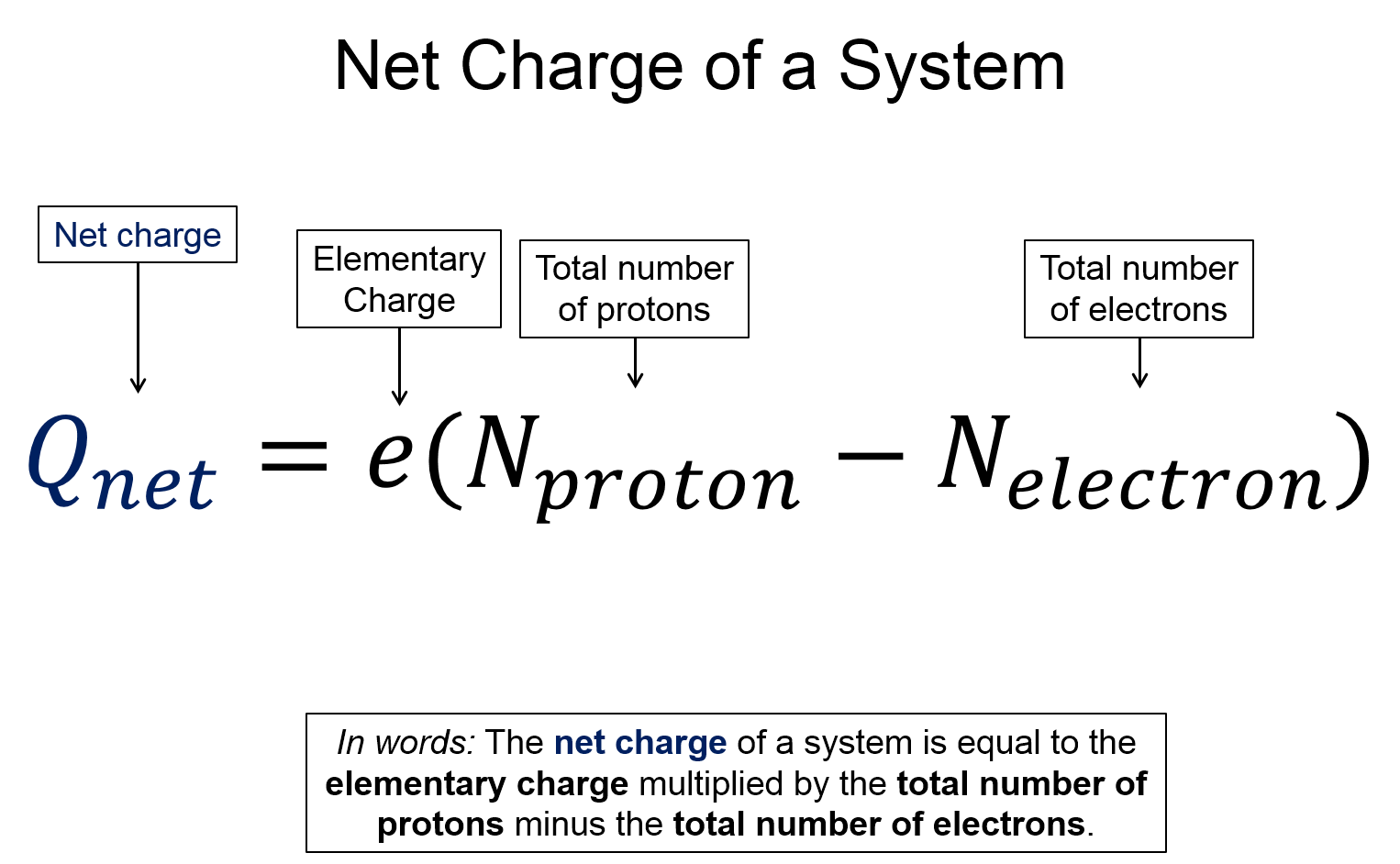
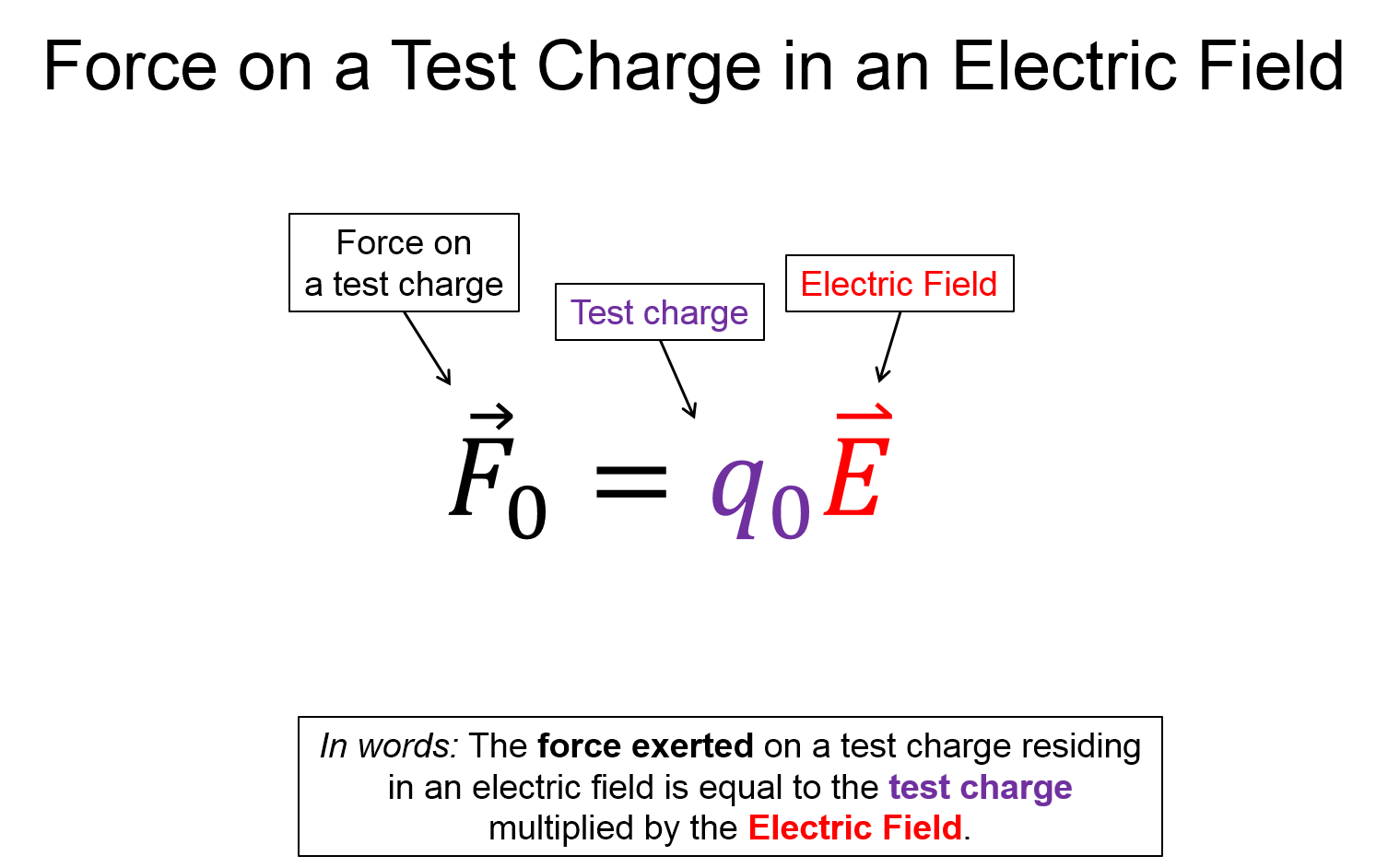
Now, take a look at the pre-lecture reading and videos below.
BoxSand Videos
Required Videos
Suggested Supplemental Videos
none
OpenStax Reading
OpenStax Section 18.1 | Static Electricity and Charge: Conservation of Charge
OpenStax Section 18.2 | Conductors and Insulators
Fundamental examples
(1) A point charge with charge $q_1 = 1.8 nC$ is located at the origin. A point charge with charge $q_2 = 3.6 nC$ is located a distance $r_{12} = 6 nm$ away. (a) What is the magnitude of the coulomb force from charge 1 on charge 2? (b) Is this an attractive or repulsive force?
(2) A point charge with charge $q_1 = - 1.8 nC$ is located at the origin. A point charge with charge $q_2 = 3.6 nC$ is located a distance $r_{12} = 6 nm$ away. What is the magnitude of the coulomb force from charge 1 on charge 2?
(3) A point charge with charge $q_1 = - 1.8 nC$ is located at the origin. A point charge with charge $q_2 = 3.6 nC$ is located a distance $r_{12} = 6 nm$ away. A point charge with charge $q_3 = 5 nC$ is located at a position $r_3 = <2,2> nm$. What is the magnitude of the coulomb force from charge 1 on charge 2?
Solutions found HERE
Short foundation building questions, often used as clicker questions, can be found in the clicker questions repository for this subject.
Post-Lecture Study Resources
Use the supplemental resources below to support your post-lecture study.
Practice Problems
BoxSand practice problems - Answers
BoxSand's multiple select problems
BoxSand's quantitative problems
Recommended example practice problems
- OpenStax, Period and Frequency also linked in Charges & the Electric Force sections.
- Static Electricity and Charge, Website Link
- Conductors and Insulators, Website Link
- Coulomb's Law, Website Link
- Large set of questions on electric charge and force. External PDF
- Coulomb's Law Review, External PDF
For additional practice problems and worked examples, visit the link below. If you've found example problems that you've used please help us out and submit them to the student contributed content section.
Additional Boxsand Study Resources
Additional BoxSand Study Resources
Learning Objectives
Summary
Summary
Atomistic Goals
Students will be able to...
YouTube Videos
Pre-Med Academy is back with three well put together videos on our main subjects, Electric Charge, Electric Force, and Coulomb's Law
Other Resources
This link will take you to the repository of other content related resources .
Simulations
This simulation by Duffy helps you with Coulombs Law and Newton's 2nd law
The colorado PhET simulation is everything you ever needed in life, that you didn't know that you needed. We present to you, John Travoltage.
Charge transfer PhET simulation with baloons and a sweater.
For additional simulations on this subject, visit the simulations repository.
Demos
Rub a plastic cup against carpet, hair, or fur, and observe the effects of the net charge now on the cup.

For additional demos involving this subject, visit the demo repository
History
Oh no, we haven't been able to write up a history overview for this topic. If you'd like to contribute, contact the director of BoxSand, KC Walsh (walshke@oregonstate.edu).
Physics Fun
Surface tension is a polarization force between atoms. It's responsible for holding a water droplet together.

A charged balloon, rubbed against hair, will gain a negative net charge (while the hair becomes positively charged) in accordance with the triboelectric series. It can then polarize and attract a neutral stream of water.

Play with John Travolta's leg and arm to get a Travoltage.
Other Resources
Boston University's Page on electric charge and Coulomb's law is neat reference with a couple of example problems
PPLATO is a complete resource with a lot of information, and several practice questions per subject. This webpage covers electric charge, the electric field, and electric potential, frocus on the electric charge, we'll get back around to field and potential later.
The Physics Classroom has an entire chapter on static electricity. The last lesson of the chapter is on Electric Fields so save that for later!
Resource Repository
This link will take you to the repository of other content related resources .
Problem Solving Guide
Use the Tips and Tricks below to support your post-lecture study.
Assumptions
Checklist
Misconceptions & Mistakes
- If you have two charges QQ and qq, and the magnitude of charge QQ is greater than that of charge qq, the two charges still apply an equal and opposite force to each other. Regardless of the relative magnitudes, Newton's 3rd Law still holds.
- While you can induce a dipole in both insulators and conductors, the atomic nature of the dipole is different for the two cases.
Pro Tips
Multiple Representations
Multiple Representations is the concept that a physical phenomena can be expressed in different ways.
Physical
Mathematical
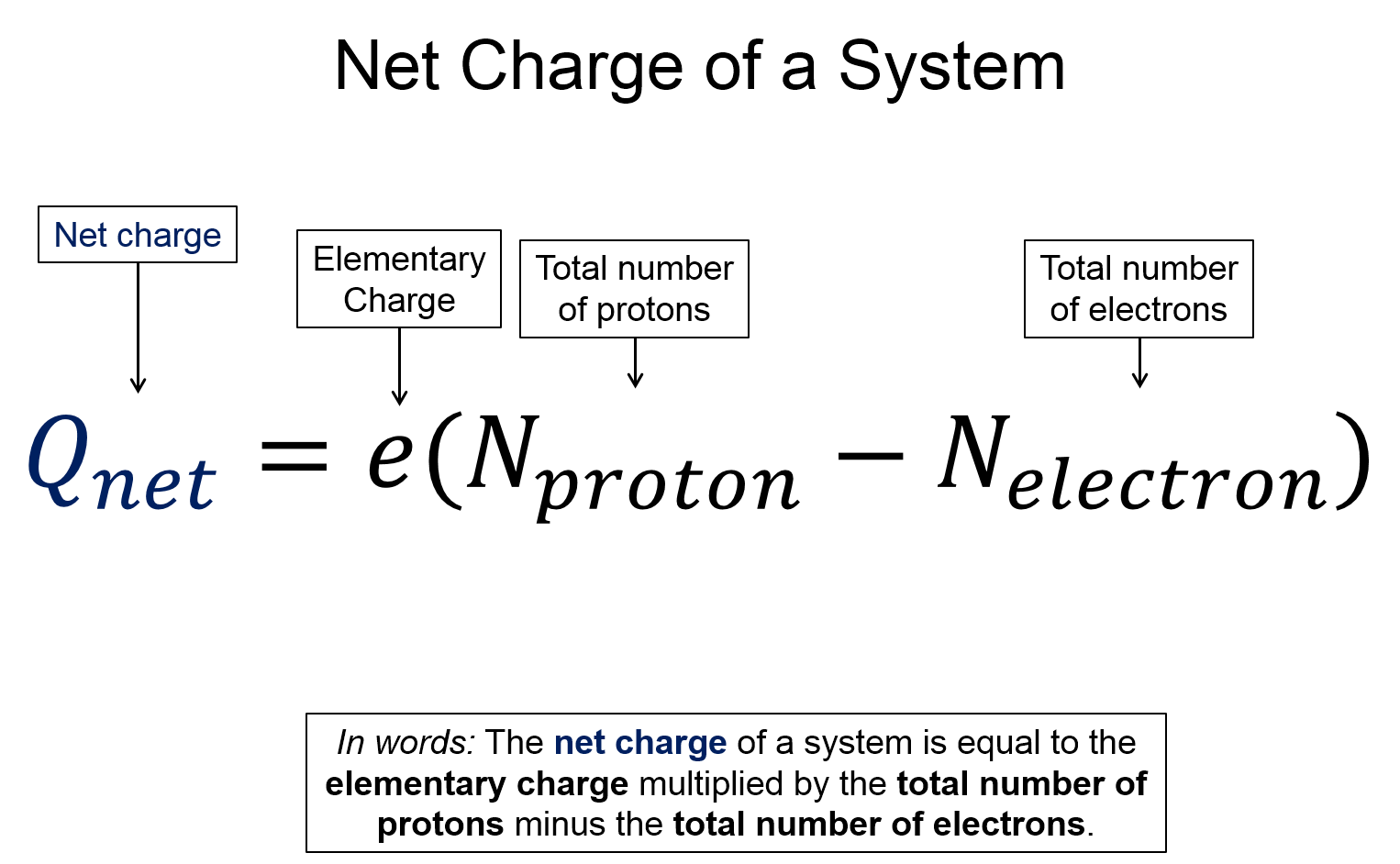



Graphical
Descriptive
Experimental