PV-Diagrams chart the change in pressure and volume of some gas as it undergoes some thermodynamic process. They are typically a plot with pressure on the vertical axis and volume on the horizontal. They show transitions from one state ($P_i, V_i$) to another state ($P_f, V_f$).
Here is a video to illustrate an object undergoing non-Uniform Circular Motion.
Pre-lecture Study Resources
Watch the pre-lecture videos and read through the OpenStax text before doing the pre-lecture homework or attending class.
BoxSand Introduction
Thermo | PV Diagrams and Ideal Gas Processes
PV-Diagrams are a useful tool for understanding thermodynamic processes. These diagrams are typically shown with pressure along the vertical axis and volume along the horizontal.

If you had an ideal gas for instance ($PV=Nk_BT$), and the number of molecules was known, then for a given pressure and volume, there would be a unique temperature. So a point on a PV diagram tells you a great deal about the state of the system. Similarly a change from one point to another point tells you a great deal about the changes in state variables and the energy transfers during that transition.

In the above diagram the pressure and volume both decreased. Assuming the number of particles in your system remained constant (an assumption always made here), with P and V both decreasing, than so must T according the ideal gas law. So this diagram helps you understand that the temperature decreased during this transition. Since internal thermal energy is related to temperature, you also know that $E_{th}$ has decreased. You can also know information about the work during this process.
Work is equal to the area under the PV-diagram curve
work is positive if the volume decreases and negative if the volume increases
So the you know the sign of the work is positive in this case, meaning the environment did work on your system. Think about compressing a gas, you'd have to push the gas into a smaller volume. You would lose energy doing the compression, which means you (the environment) is losing energy (Wenvironment is negative) so the system (the gas) is gaining that energy and thus Wsystem is positive. Put all that together with the first law of thermo $\Delta E_{th}=Q+W$: you know that $\Delta E_{th}$ is positive, and so is W, so what is the sign of the heat Q and how large is it? In this case Q could be positive or negative and to find the magnitude, you'd have to calcuted the change in thermal energy and the work, then use the first law to solve for Q. If $\Delta E_{th}$ is a larger positive than W, then Q must be postive. If $\Delta E_{th}$ is a smaller positive than W, then Q must be negative.
It's important to note that there are other important thermodynamic curve diagrams, pressure vs. temperature or temperature vs. volume among others, but we have decided to focus only on the PV-diagrams here.
Ideal Gas Processes
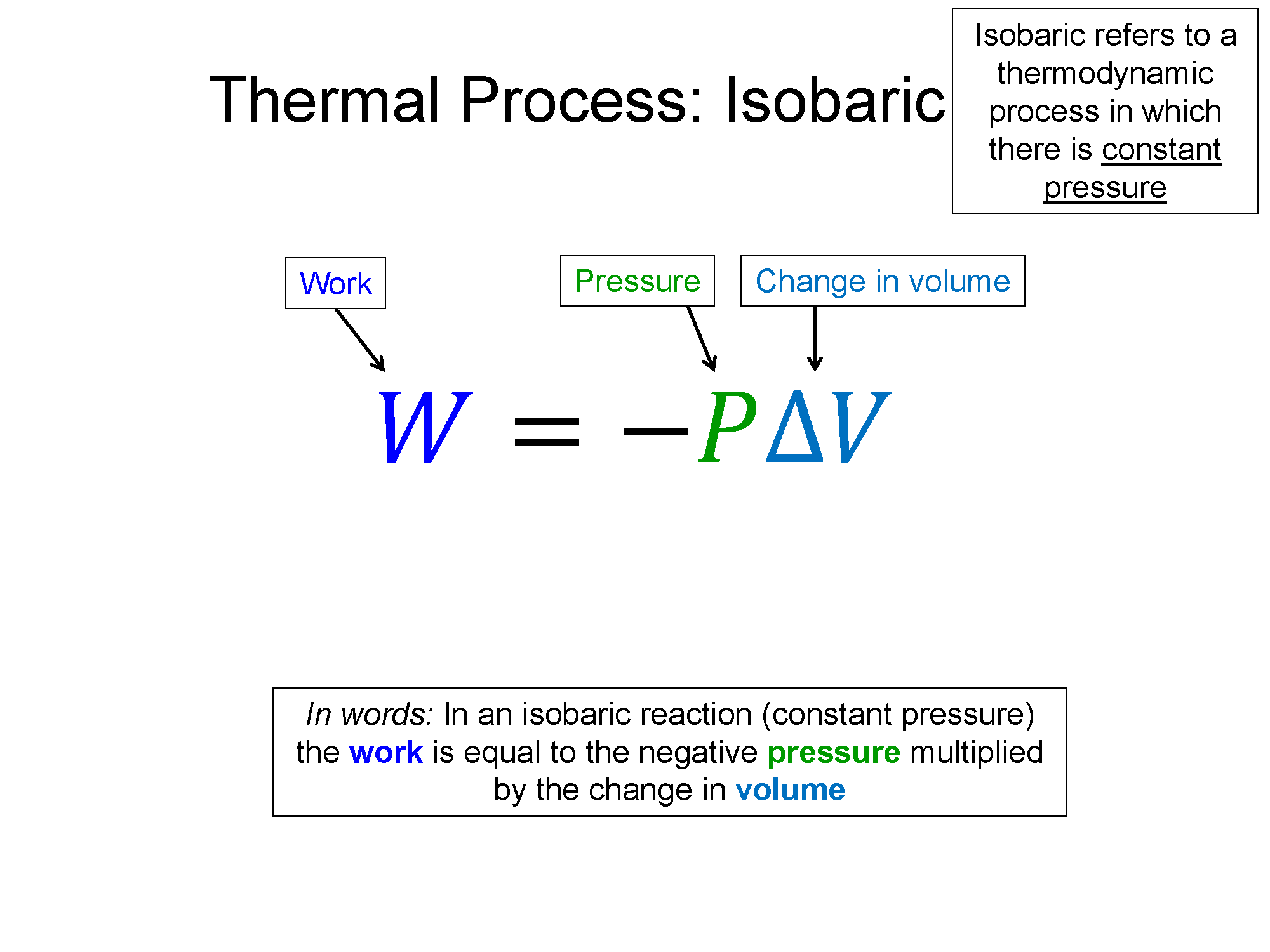
Isobaric: Pressure is sometimes measured in a non-SI unit called Bars. Iso is a prefix meaning constant. So, isobaric refers to a constant pressure process.

Work = area under PV-curve, for isobaric: $W=P \Delta V$
With pressure constant and volume increasing, the temperature must also increase. Since the temperature increases, so does the thermal energy. In an expansion, like that shown above, the work is negative. With the first law ($\Delta E_{th}=Q+W$), heat (Q) is equal to the the change in thermal energy minus the work. With a positive $\Delta E_{th}$ and negative work (W), heat (Q) must be positive and greater in magnitude than the work. So in an isobaric expansion, energy goes in via heat more quickly than it goes out via work and thus the thermal energy increases.
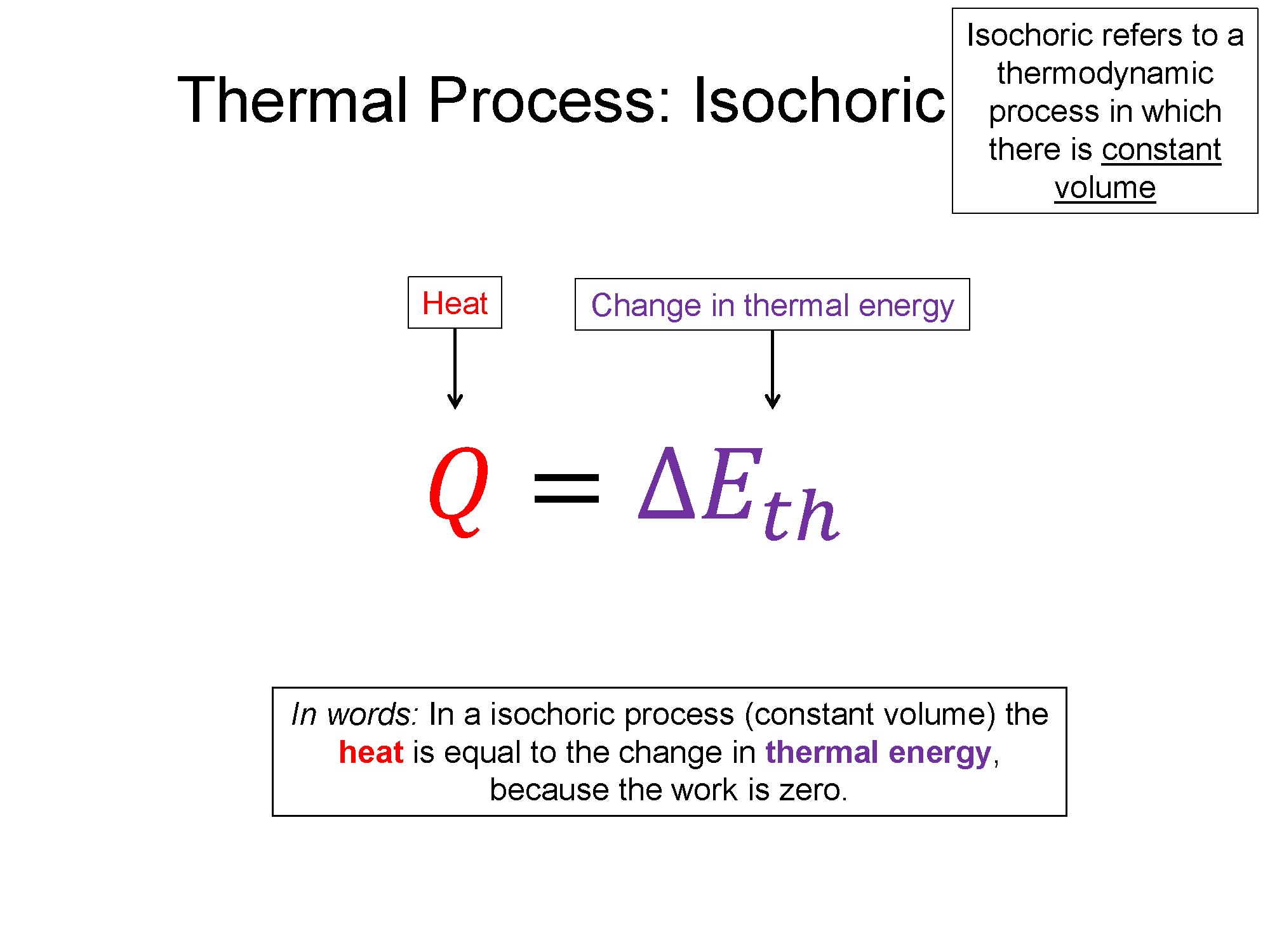
Isochoric: A process where the volume remains constant is considered an isochoric process. From the ideal gas law, if the volume remains constant but the pressure increases, like in the diagram below, then the temperature must increase.

Work = area under curve, w/ $W = 0$, $Q = \Delta E_{th}$
Since work is the area under the PV-curve, the work in an isochoric process is zero. With work equal to zero, the heat is equal to the change in thermal energy (1st law). Since the temperature increased in the above diagram, the thermal energy must also have increased. Heat entered the system while zero work was done by the system, as a result it increased its temperature.
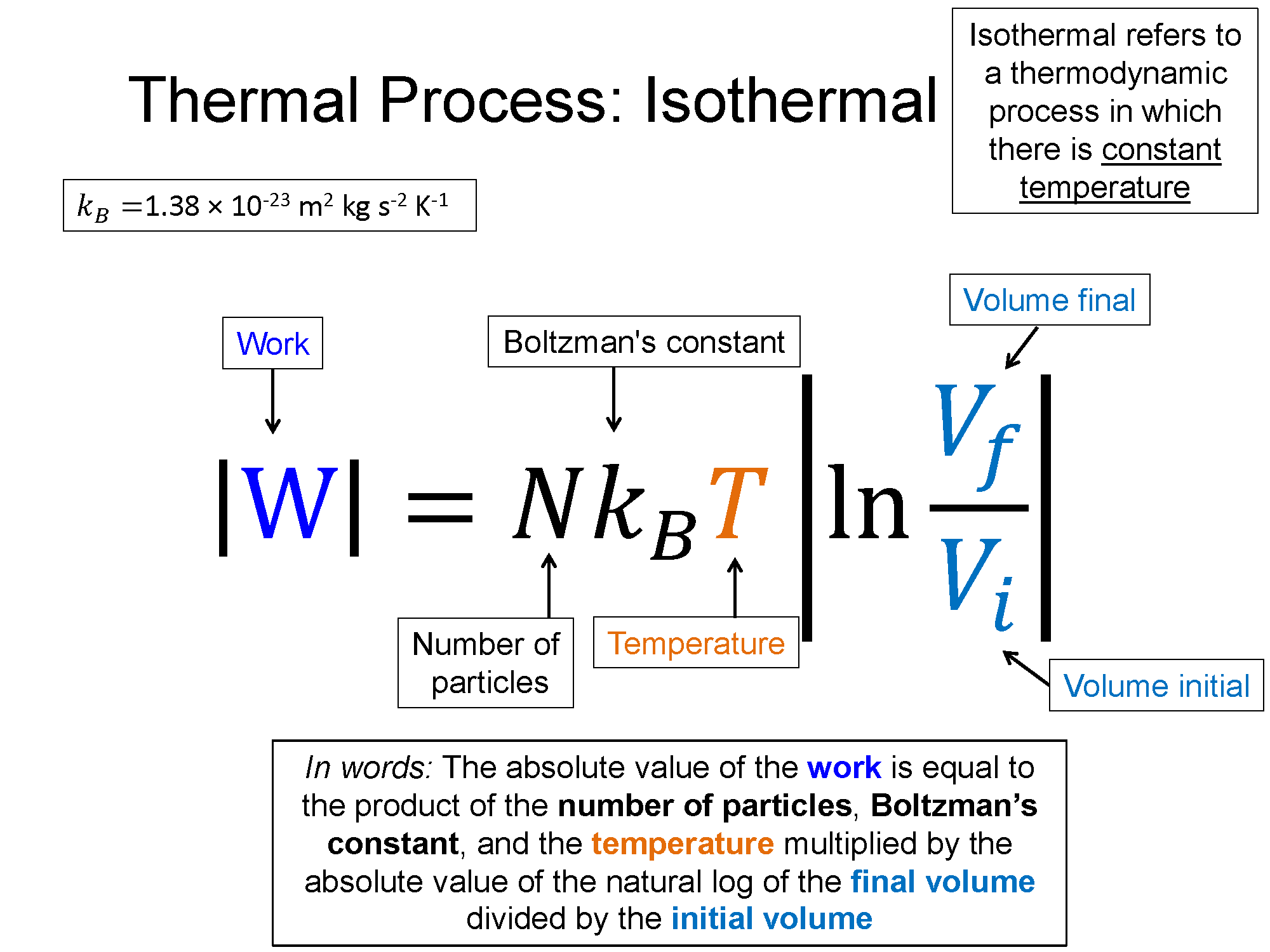
Isothermal: A process where the temperature remains constant is considered an isothermal process. The ideal gas law shows us that if the temperature is constand the volume increases, then the pressure must decrease. Because of the inverse relationship between pressure and volume when temperature (and number) is constant, isothermal transitions are always curvy lines in a PV-diagram.

Work = area under curve, for isothermal: $|W|=Nk_BT |\ln{\frac{V_f}{V_i}}|$
In an isothermal process the internal thermal energy remains constant, so from the 1st law, the heat must be equal to negative the work. If the system is expanding, like that shown above, the work is negative so the heat must be positive. Heat is entering into the system at the same rate work is taking that energy out of the system.
Adiabatic: A process where no heat is transfered into or out of the system is considered an adiabatic process. On a PV-diagram adiabatic curves are curvy like isotherms, but they are steeper.

Work = area under curve, w/ $Q = 0$, $W = \Delta E_{th}$
In an adiabatic process there is no explicit equation for the work in terms of the state variables. In these transitions, you can use the first law and the fact that the heat is zero to find the work equal to the change in internal thermal energy. For a monoatomic gas we know that $\Delta E_{th}=\frac{3}{2}Nk_B \Delta T$, an equation found in the kinetic theory of gases.
Key Equations and Infographics
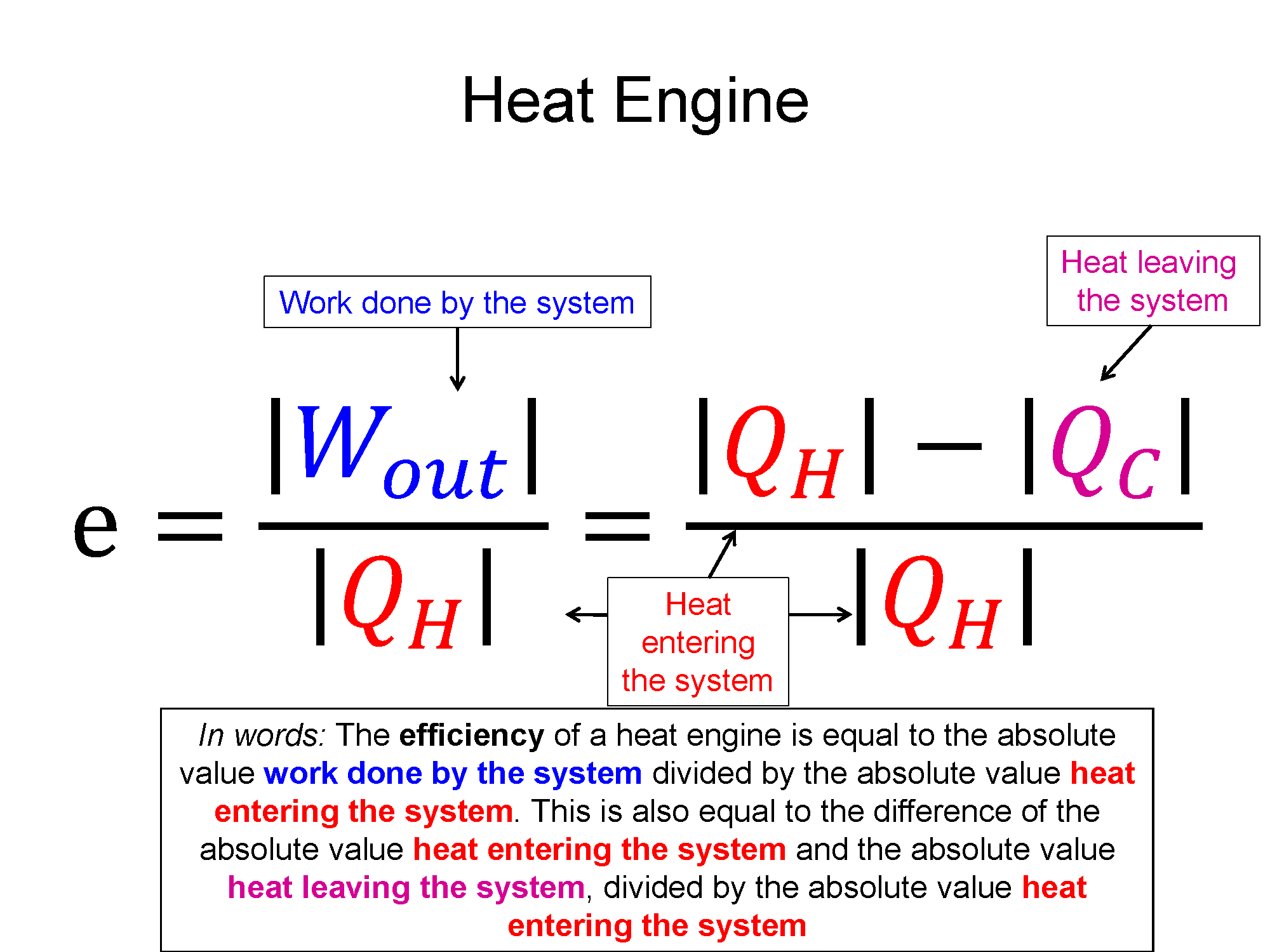
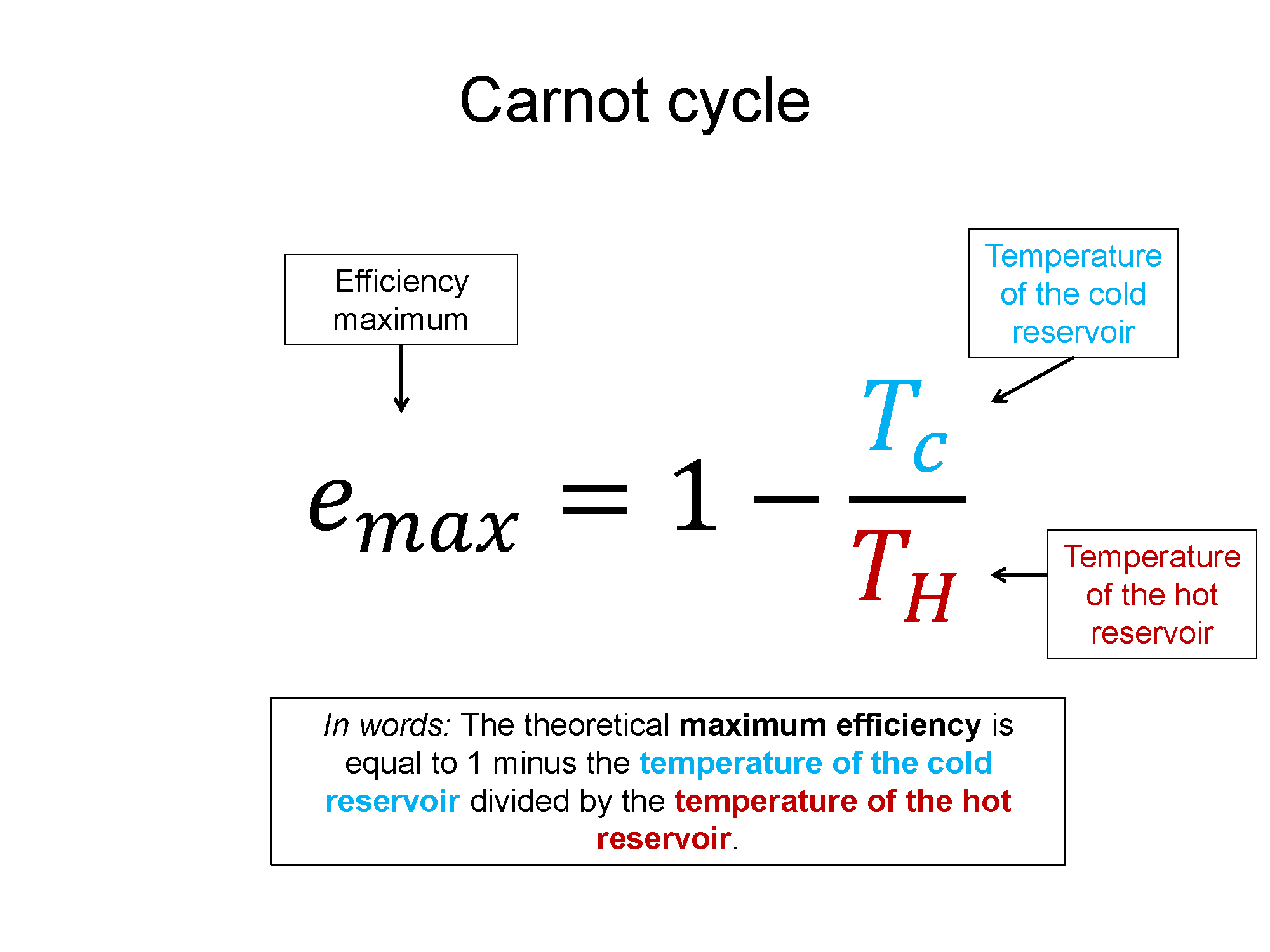
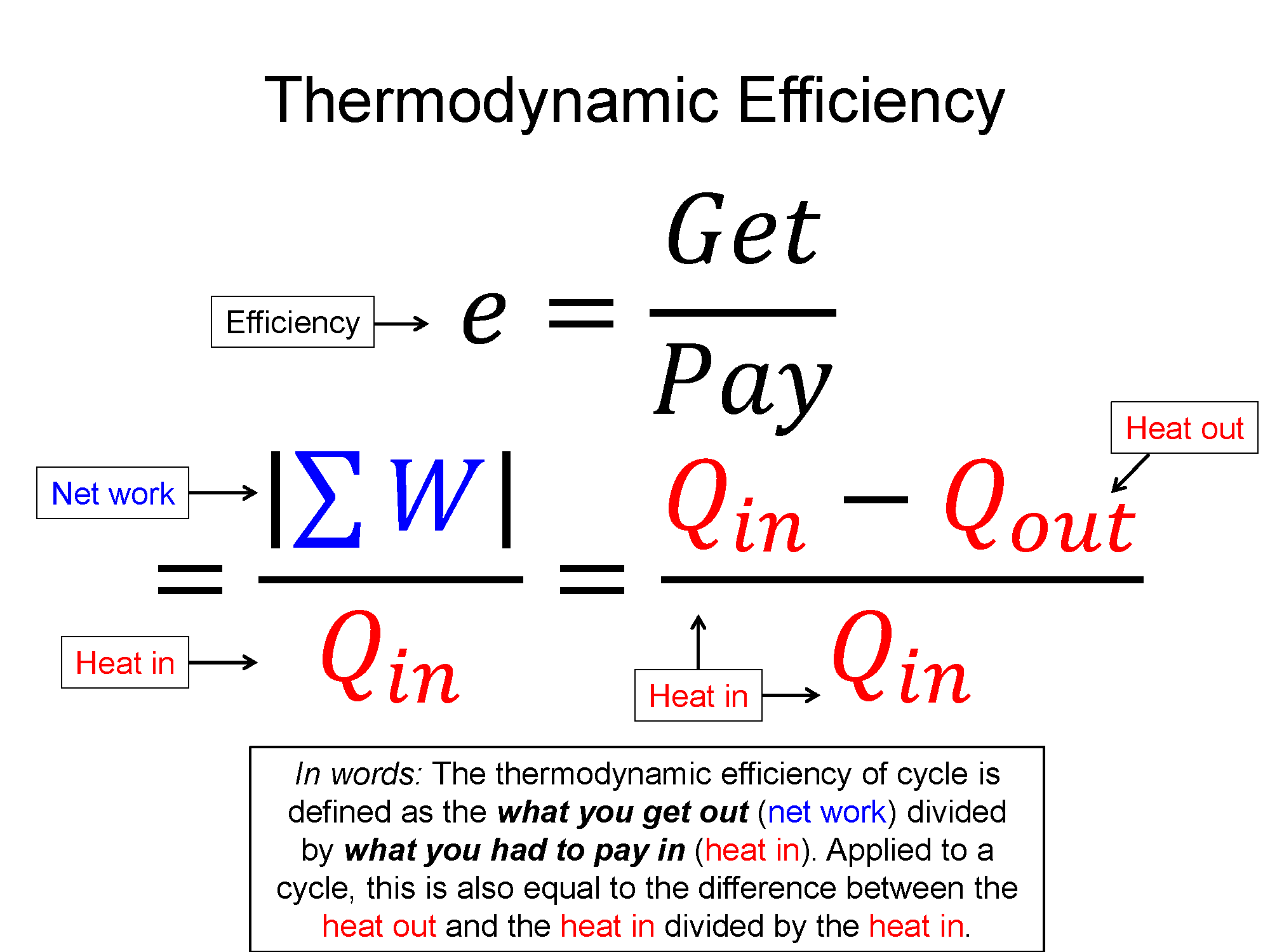
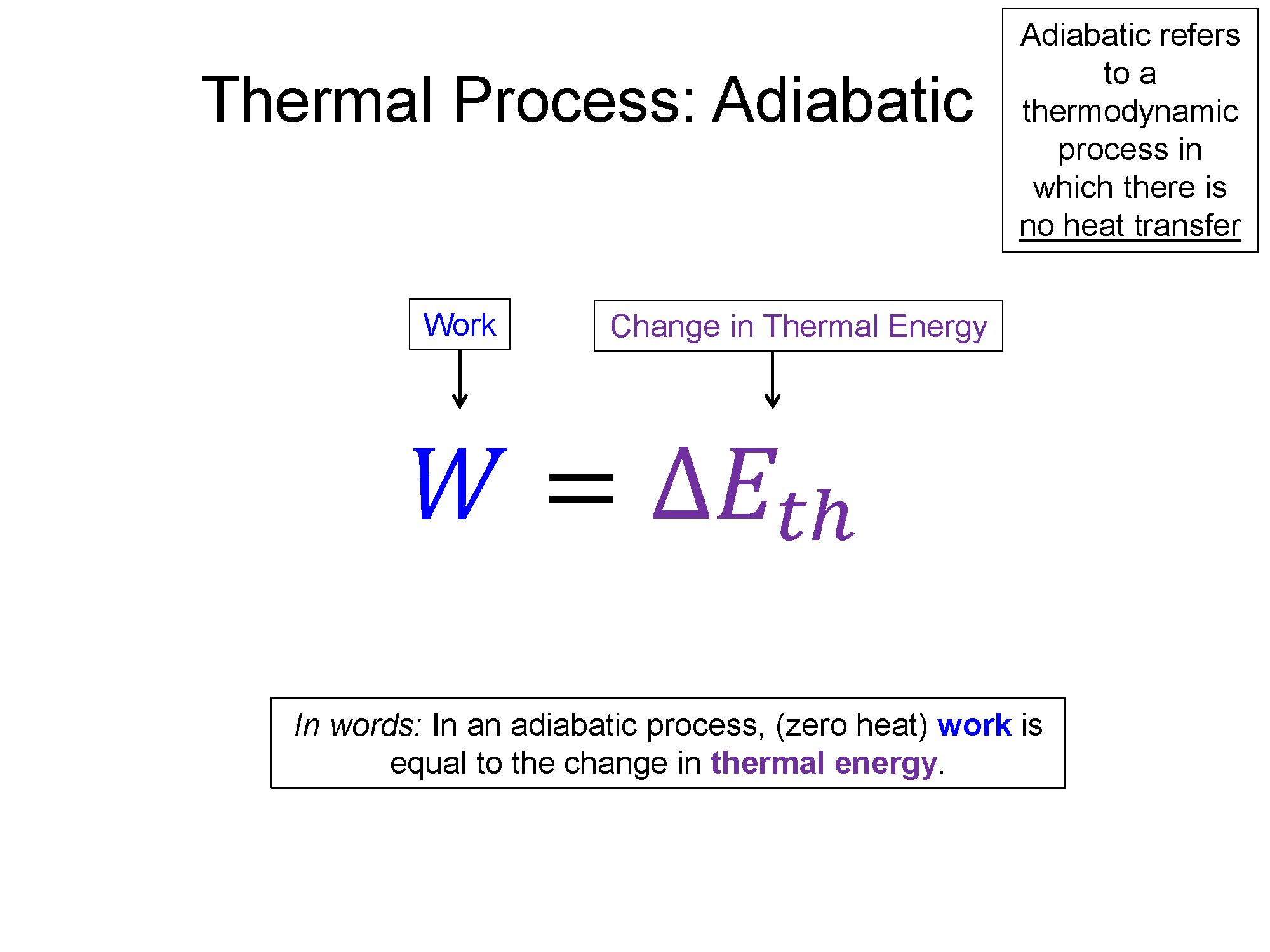
Now, take a look at the pre-lecture reading and videos below.
BoxSand Videos
Required Videos
Ideal gas processes overview(3min)
work gas expansion compression(5min)
ideal gas processes isochoric(3min)
ideal gas processes isobaric(2min)
ideal gas processes isothermal(3min)
Suggested Supplemental Videos
none
OpenStax Reading
OpenStax Section: none
Fundamental examples
1. (a) and (b): which type of thermodynamic process is sketched? (c) Sketch the thermodynamic process in which temperature does not change. (d) Sketch the thermodynamic process in which heat neither enters or nor leaves the system.

2. During a thermodynamic process, the pressure in a container goes to $\frac{2}{3}$ it's original pressure: $P_f = \frac{2}{3} P_i$. Likewise, $T_f = 2T_i$. (a) What is the final volume (in terms of $V_i$)? (b) Is $\Delta E$ for this process positive or negative? (c) Does heat enter or leave the system during this process, and if so, in which direction does heat flow?
3. Using the diagram below, find the following: (a) the temperature $T_1$ at point 1 and the pressure $P_3$ at point 3. (B) For each step of the process - from points 1 to 2 and from points 2 to 3 - was the change in thermal energy positive or negative? The work? Did heat enter or leave the system during this stage, and how much? Hint: make a table.
Short foundation building questions, often used as clicker questions, can be found in the clicker questions repository for this subject.
Post-Lecture Study Resources
Use the supplemental resources below to support your post-lecture study.
Practice Problems
BoxSand's multiple select problems
BoxSand's quantitative problems
Recommended example practice problems
- The Openstax section for has several practice exercises at the bottom of the page, Website Link
- Large set of pv diagram related problems, answer key at the bottom, PDF Link
For additional practice problems and worked examples, visit the link below. If you've found example problems that you've used please help us out and submit them to the student contributed content section.
Additional Boxsand Study Resources
Additional BoxSand Study Resources
Learning Objectives
Summary
Summary
Atomistic Goals
Students will be able to...
YouTube Videos
These videos discuss first what is a PV-diagram, and then how to use a PV-Diagram to determine the work done on the sytem for the four types of processes
This Video is a continuation of the previous and focuses on Isothermal Isometric and Adiabetic processes,
Other Resources
This link will take you to the repository of other content related resources .
Simulations
Here is a link to PCCL's basic simulation demonstration the connection between pressure and volume
For additional simulations on this subject, visit the simulations repository.
Demos
History
Oh no, we haven't been able to write up a history overview for this topic. If you'd like to contribute, contact the director of BoxSand, KC Walsh (walshke@oregonstate.edu).
Physics Fun
Other Resources
Boston University's page for the first law of thermodynamics discusses the use of pv-diagrams.
This PDF covers PV-Diagrams and the four process types, and how to find the work done,
Hyperphysics's reference page for PV diagram snad heat cycles,
![]()
This link will take you to the repository of other content related resources .
Resource Repository
This link will take you to the repository of other content related resources .
Problem Solving Guide
Use the Tips and Tricks below to support your post-lecture study.
Assumptions
There are a LOT of things going on in analyzing a thermodynamic cycle. Here are some things that are always true to help you:
Remember the names of processes and tie them to their meaning. Iso- is a prefix that means "same", if it is there, that means the word that follows it is held constant in that process. Isothermal means process with constant temperature, isobaric means process with constant pressure, isochoric means process with constant volume. Also remember the shapes that go with each (check out the bakc of the physics survival sheet for this section for the most useful table for these processes: Survival Sheet.
There are a number of easily identifiable 0's that show up when analyzing thermodynamic cycles, here are the most frequent ones:
1) Delta Eth (change in thermal energy) for a cycle that returns to where it starts is always 0!
2) Delta Eth for an Isothermal process is always 0! This always means for an isothermal process W = -Q!
3) Work (W) for a isochoric process is always 0 (vertical line with no area underneath on a PV diagram means W = 0)!
4) Heat in an adiabatic process is always 0.
When drawing a PV diagram, an Isothermal curve must ALWAYS curve AWAY from the P and V axes (or get closer to them as P or V increases if you prefer to think of it that way). It is a frequent mistake since isothermal curve are parabola-like and bow towards or away from the origin, but they must ALWAYS bow towards the origin (open away from it).
The 1st law is always true, both for the total cycle and each step along the way, use it everywhere to save a lot of time and energy once you know 2 out of 3 of work, heat, or change in thermal energy in any section of the cycle.
The area underneath the curve in a PV diagram is work! Use geometry to find it unless the curve is isothermal, then use the equation for it!
There are two ways to tell a heat engine from a heat pump/refrigerator 1) If it is a cycle (returns to the place it starts on a PV diagram) if it progresses clockwise then it is a heat engine, counter-clockwise is a heat pump/refrigerator. 2) Heat engines do net work, heat pump/refrigerators take in net work. Therefore, you can tell by the sign of the work, negative is a heat engine, positive is a heat pump/refrigerator. Without calculating anything, you can tell this by finding the sign of work for the process on the top of the diagram (has most area underneath it, so it determines the sign of work). For expanding processes where V increases, work is negative. For contracting processes where V decreases, work is positive.
Checklist
For any thermodynamic cycle:
1) Breakdown how many different processes there are and what type each is (isobaric, isothermal, isochoric, etc.)
2) Draw the PV diagram for the cycle
3) Make a table where the rows are each process it undergoes and the columns are for work, heat, and thermal energy.
4) Start where you know the most information in the cycle (usually where you know 2 out of 3 of P, V, and T), and begin using your equations and geometry to fill out the work calculations, then find heat and Eth afterwards depending on which are easier. Remember, you know Delta Eth for the entire cycle is zero, and for every process you can apply the 1st law (Delta Eth = Q + W), so you should only ever need to know 2 out of 3 of Delta Eth, W, and Q for any process and can use simple addition and subtraction with the 1st law to find the last one!
Misconceptions & Mistakes
-
Not making a table
-
Forgetting which equations you have at your disposal: first law, second law, equipartition theorem, and ideal gas law. (I think this is an exhaustive list..? I'll watch lecture vids tomorrow and see if more come up)
Pro Tips
-
Make a table! You will be so lost without one. A table helps you identify which information you have and what you lack. It will also make the relationships between different steps more obvious - recall when doing energy analysis, we could often fill in "potential energy = 0" to multiple entries. The same thing happens with PV Diagram tables. This helps you determine which variables you have are actually unkown, which helps you find the correct equations to apply.
-
PV Diagrams are tedious, don't expect them to be anything else when digging into one. You will not have a linear path to solving the problem (you probably can't just go left-to-right and top-to-bottom filling out your table) and will probably have to jump between different points in the cycle. This is okay, this is how they're supposed to be done.
-
Lots of systems change their thermal energy by units of $\frac{3}{2}Nk_{B}T$, and the thermal energy of a system is often $\frac{3}{2}Nk_{B}T$. For lots of book problems/mastering physics problems/homework problems, the entry in one of the boxes is just $\frac{3}{2}Nk_{B}T$, where T is the temperature at the point (probably labeled with a subscript so $T_A$, $T_B$, or something like that). Get used to seeing and writing $\frac{3}{2}Nk_{B}T$.
-
The only equations you need are the ideal gas law, the first law, the second law, and the equipartition theorem. A few of these combine to make the virial equation, which is also sometimes useful.
-
$Nk = nR = pV$. That means $3/2 nKT = 3/2 pV$. This is sometimes useful <needs formatting>
Multiple Representations
Multiple Representations is the concept that a physical phenomena can be expressed in different ways.
Physical
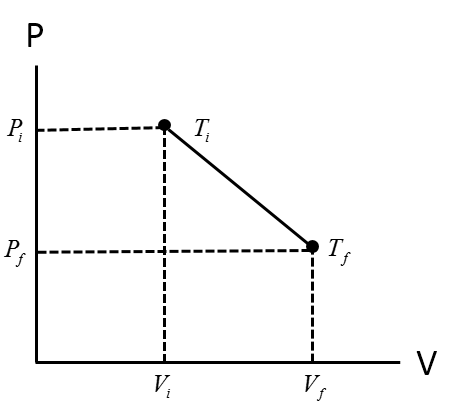
The graphical nature of the PV diagram describes the relationship between pressure and volume with respect to the state temperature.
Mathematical
$PV=nRT = N K_b T$
Pressure ($P$), Volume ($V$), Numer of mols ($n$), Rydberg constant ($R$), Temperature ($T$), Numer of particles ($N$),
Boltzman constant ($K_{b}$)




Graphical
The graphical nature of the PV diagram describes the relationship between pressure and volume with respect to the state temperature.

Descriptive
PV diagrams are directly related to the ideal gas law which describes the relationship between pressure (P), volume (V), and temperature (T). Namely, pressure and volume are inversely proportional to each other when the temperature is held constant. In addition, there is a direct relationship between pressure and temperature when the volume is held constant, or conversely there is a direct relationship between volume and temperature when the pressure is held constant.
Experimental
A quick experiment could be to measure the pressure of a piston relative to the volume inside. As we can see from the graph, when the temperature is held constant at say $T_{i}$, then we can see the explicit inverse relationship between pressure (P) and volume (V).