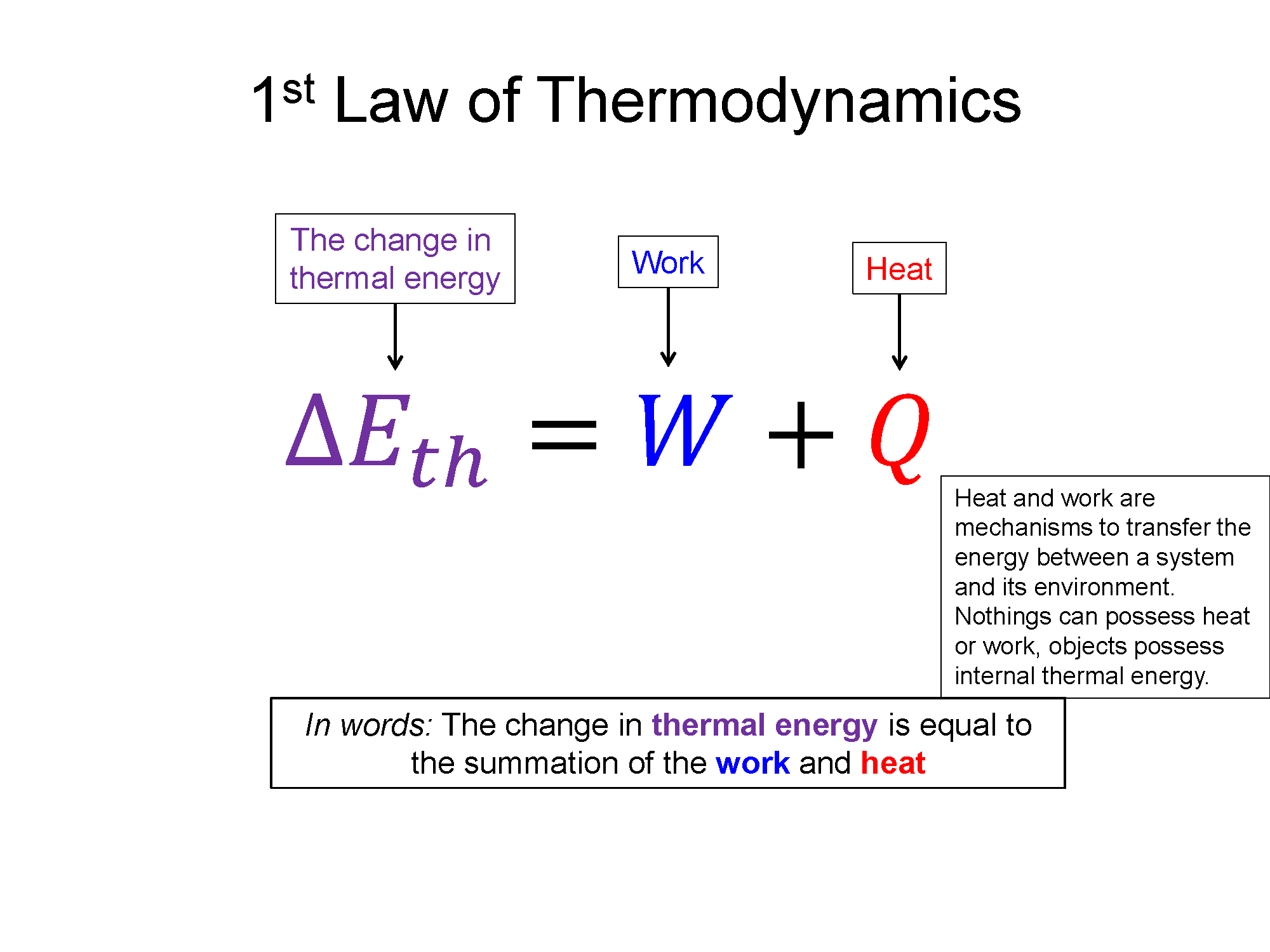
The First Law of Thermodynamics is one we talk about often. It is an extension of the Conservation of Mechanical Energy previously learned. It states that the change in thermal energy (Eth) of a system can be done via two mechanisms, heat transfer (Q) and work (W).
ΔEth=Q+W
An introduction to the first law of thermodynamics by the Royal Institute
Pre-lecture Study Resources
Watch the pre-lecture videos and read through the OpenStax text before doing the pre-lecture homework or attending class.
BoxSand Introduction
Thermo | 1st Law of Thermodyamics, Energy Transfers
If you recall the conservation of mechanical energy stated that work (W) is a mechanism for changing the energy of a system.
Ei+W=Ef, which can be rearranged to state, W=ΔE
Although this is sufficient to study mechanics, it falls short when thermal energy is involved or when energy is transferred via heat - stand next to a fire and you can feel the difference in the energy when compared to far away. It is the heat (Q) transfer you're feeling that increases your internal thermal energy. The Kinetic Theory of Gases introduces thermal energy (Eth) as proportional to the temperature. So the first thing we need to do is expand our energy model to include thermal energy. (In fact there are other energies that we will add later, such as the energy to create/annihilate a particle, Einstein's's famous E=mc2)
Etotal=Klinear+Krotational+Ug+Us+Eth+...
Now with this expanded view of energy we can imagine not only changing the energy of the system via work, but also through heat. The first law of thermodynamics is this energy transfer statement: ΔEtotal=Q+W. Now since most thermodynamic problems do not involve using heat to change the collective kinetic or potential energy of a system but rather the temperature (microscopic random kinetic energies of the individual particles), the only relevant energy is the thermal energy. So the most common form of the first law of thermodynamics is the following.
ΔEth=Q+W
What's incredibly important here is that nothing possess heat or work, they possess internal thermal energy. Heat and work are simply mechanisms to transfer the energy between a system and its environment. Colloquially people often say things like, "that fire's got a lot of heat", which in a physics view is an incorrect statement. The fire contains a lot of thermal energy, as shown by the high temperature, and it is transferring a lot of that energy to the environment via the mechanism of heat. If the environment the fire is in is at the same temperature, there would be zero heat transfer. An example of using work to change the thermal energy of a system would be rubbing your hands together and feeling them warm up.
Key Equations and Infographics
Now, take a look at the pre-lecture reading and videos below.
BoxSand Videos
Required Videos
Suggested Supplemental Videos
OpenStax Reading
OpenStax Section 15.1 | The First Law of Thermodynamics
** Warning: OpenStax uses an alternate form of the 1st law that has a minus sign difference from the one used in our class. To clear up any confusion, please see the BoxSand Video below about alternative forms of the 1st law. **
Fundamental examples
1. Assume a delicious, delicious lamb gyro from Crystal's Cuisine & Cafe has 800 Calories. How much energy is this in joules?
2. You have an insulated container of 6.022×1023 monatomic particles. During a thermodynamic process, the system is compressed. This results in 2 kJ of Work being done to the gas and its temperature being raised by 250 K. a) What is the change in thermal energy of the system? b) Did heat enter or exit the system during this process, and if so, how much (magnitude and sign of Q)?
3. In a certain process 20 kJ of work is done on a system while 8 kJ of heat is extracted. If n=3 mol and the gas is monatomic, what is ΔT?
Short foundation building questions, often used as clicker questions, can be found in the clicker questions repository for this subject.
Post-Lecture Study Resources
Use the supplemental resources below to support your post-lecture study.
Practice Problems
Recommended example practice problems
- Openstax, 15.1 - 7 conceptual questions and 9 practice exercises at bottom of the page Website Link
- Two problems on the first law of thermodyanmics, PDF Link
For additional practice problems and worked examples, visit the link below. If you've found example problems that you've used please help us out and submit them to the student contributed content section.
Additional Boxsand Study Resources
Additional BoxSand Study Resources
Learning Objectives
Summary
Summary
Atomistic Goals
Students will be able to...
YouTube Videos
Doc Schuster discusses the 0th and 1st law of thermodynamics
This video from Khan Academy goes through an example problem about the first law of thermo dyanmics.
Other Resources
This link will take you to the repository of other content related resources .
Simulations
When using this PhET simulation, pay attention to how adding or removing energy from the system effects the internal energy.
For additional simulations on this subject, visit the simulations repository.
Demos
Check out these two cool 1st law demonstrations.
For additional demos involving this subject, visit the demo repository
History
Oh no, we haven't been able to write up a history overview for this topic. If you'd like to contribute, contact the director of BoxSand, KC Walsh (walshke@oregonstate.edu).
Physics Fun
Other Resources
Resource Repository
Khan Academy has an excellent walkthrough of the first law.
Hyperphysics gives a brief description of the first law of thermodynamics.
Another great overview of the first law of thermodynamics
This website does a good job explaining everything in the subject of the First Law. It will cover what an equation and property of state is, what it means for a boundary to be open or closed, and how to identify your system. There are several good example problems to go over as well. Note that in one or two places concepts from calculus are used, just ignore the calculus equations and pay attention to the words around them.
BU Physics covers the first law of thermodynamics with work.
Here is a set of lecture slides on the first law. These slides also cover the different thermodynamic processes, you can stop there as we will get to those later in the thermodynamic cycles section.
This link will take you to the repository of other content related resources .
Problem Solving Guide
Use the Tips and Tricks below to support your post-lecture study.
Assumptions
The 1st law of thermodynamics is one of the most universal and broadly applicable laws in all of physics. As such, it relies on few if any assumptions! The mathmatical formulation of it we will use: Eth = Q + W, assumes only that there is no addition or subtraction of matter from the system, but that is it!
The 1st Law essentially states that energy cannot be created or destroyed, it can only change forms. It is also the theoretical basis for the idea of conservation of energy that we explored in PH201! However, while many highly theoretical laws aren't useful for real world calculations, the 1st law very much is! It is called the 1st law for a reason, and writing down Eth = Q + W on every problem as a starting point isn't a bad idea. It is always true, and even if you do not end up using it to solve the problem, it is one of the best sensemaking tools to use in thermodynamics, so always keep it in consideration! It should be one of the first thing you think about when checking to see if your answers make sense!
Checklist
1. Problems where ΔEth=0 and thus Q=−W
2. Problems where Q=0 and thus ΔEth=W
3. Problems where W=0 and thus ΔEth=Q
Misconceptions & Mistakes
Pro Tips
- In thermodynamics, work is typically "PV work." Work has the same definition and dimensions as before - Force times change in distance - but now the "distance" is typically a changing volume or surface area, rather than a linear distance. A quick examination of the dimensions show that they're equal:
P=FA which has dimensions of [P]=[N][L2].
Volume has dimensions [L3]. Multipling them together, we see:
[N][L2]∗[L3]=[N][L]
which are the dimensions of work we recognize from previous sections.

Multiple Representations
Multiple Representations is the concept that a physical phenomena can be expressed in different ways.
Physical
The change in energy of a system is due to the amount of work and heat that goes in or out of the system.
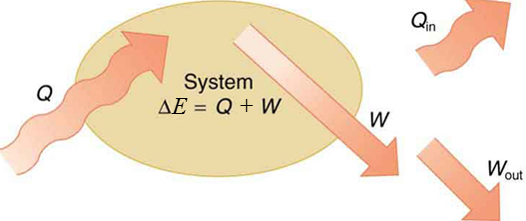
Mathematical
Q+W=ΔE
E=K+U+Eth+...
Heat (Q), Work (W), Energy (E), Kinetic Energy (K), Potential Energy (U)

Graphical
The net energy is the sum of the work and heat in the system. As soon as we increase the heat going into the system, we see the energy change occur.
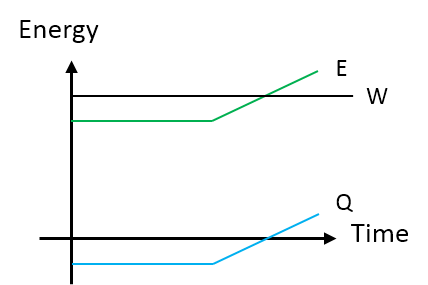
Descriptive
From the Graphical Representaion, as energy leaves the system as heat (Q) there is also constasnt work (W) being done on the system such that the energy (E) remains constant. Then, as the thermal energy (Q) of the system begins to increase with the same costant work (W), the energy (E) of the system increases.
Experimental
We can attach a balloon to an empty water bottle and submerge the empty bottle in to a bath of hot water. The hot water will excite the air molecules in the bottle which means that the temperature of the air inside the bottle has increased and therefore the energy of the system inside the bottle has increased. The increased kinetic energy of the air molecules in the bottle will inflate the balloon.